
300
Active Clients
45
Therapeutic Areas
15,000+
Global Sites
EDC & CDMS
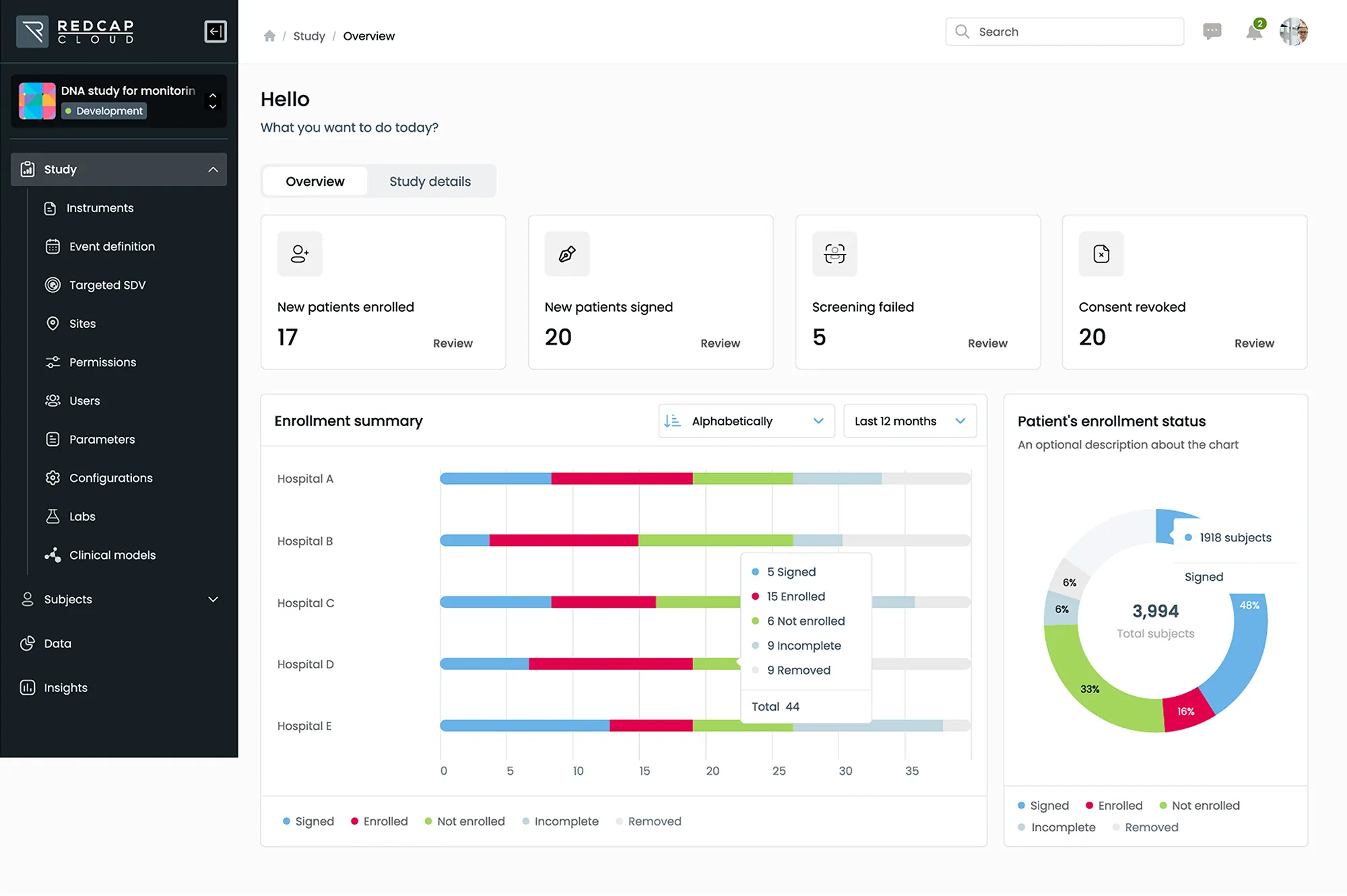
Leverage the industry’s most advanced, scalable and secure electronic data capture solution to collect and manage all data from labs, sites, patients and digital health devices.
- Manage studies of any size, complexity, or therapeutic area within a single, unified platform
- Deliver cross-functional and cross-study insights with a standards-based approach to data management and analytics
- Accelerate decision making through direct access to study data and real-time dashboards via our web-based portal or mobile EDC app
Streamline study timelines by making design changes to active studies easily without the need for migrations
The industry’s most dynamic data science platform
REDCap Cloud technology empowers organizations to easily collect, integrate, standardize, analyze, and share real-world, regulatory-grade data for any use case. Our modern platform delivers a customizable suite of integrated applications to support development of life science breakthroughs from research to commercialization.
How it Works
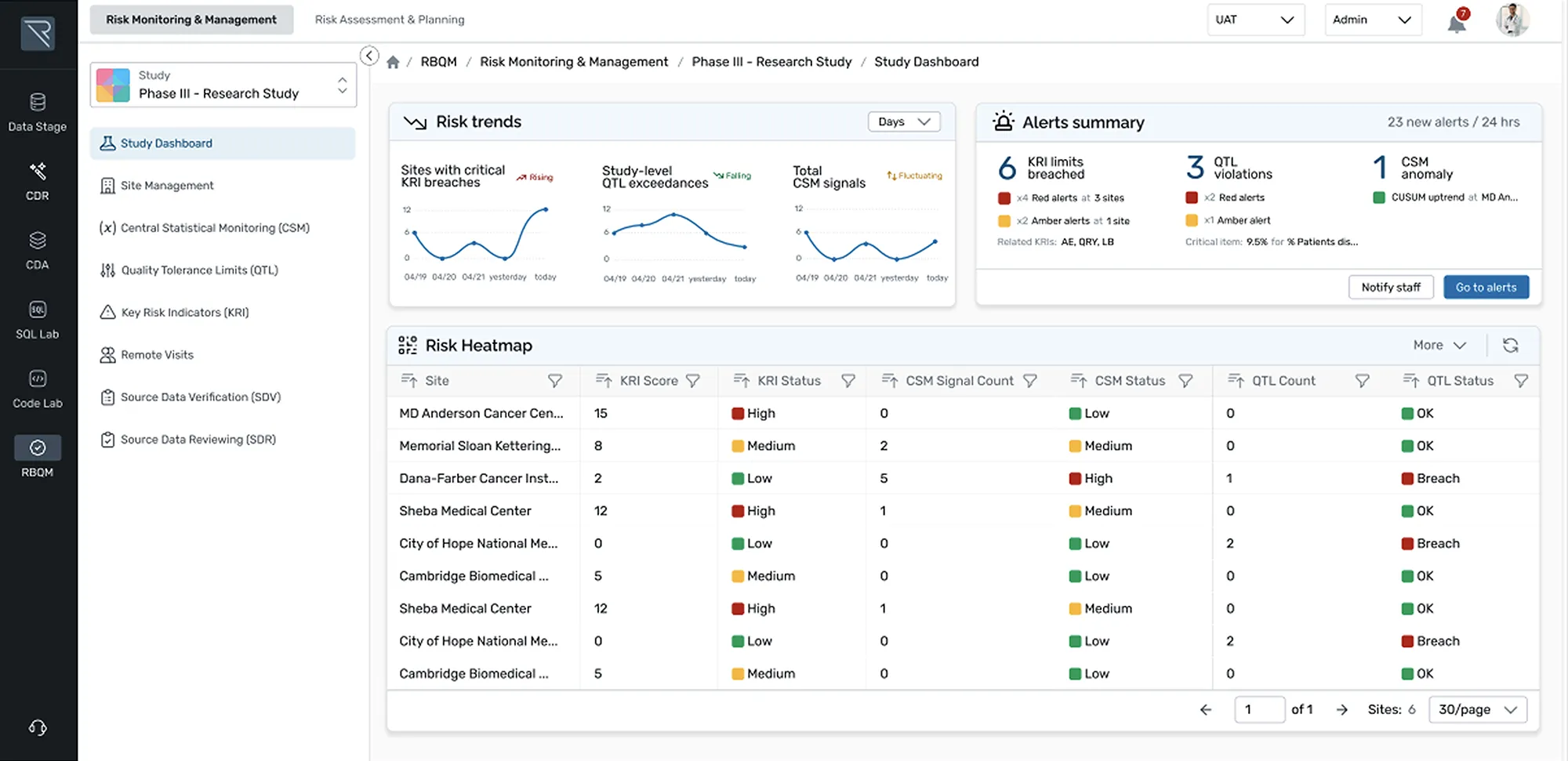
Designed for ease and efficiency, REDCap Cloud is an all-in-one data management and collection platform that lets you securely build, collect, manage, and visualize studies in real-time.
- Create: Create custom screening tools to capture your desired cohort
- Automate: Use rules and expressions to trigger automatic CRF delivery
- Standardize: Use medical coding libraries to ensure use of standardized terminology for clinical data
- Save & Reuse: Save your study as a global template for future use
REDCap Cloud Solutions
Study Central
Design and deploy adaptive trials, registries, and everything in between. Integrate hundreds of clinical and RWD sources while leveraging global libraries and custom functions to ensure consistency across the entire trial life-cycle.
Safety Gateway
Automate the collection, tracking, and notification of both adverse events and serious adverse events in real-time
Reporting and Analytics
Leverage the most advanced purpose-built Statistical Computing Environment(SCE) to perform cross study analytics and ML/AI analysis in real-time.
Patient Engagement
myREDCap Cloud is a comprehensive solution for hybrid or decentralized clinical trials that simplifies patient enrollment and participation throughout the clinical trial process.
Medical Coding
Expedited and accurate coding supported by integrated workflows that enable user or auto populating of preferred medical terms from a range of medical dictionaries
Interoperability & RWE
Seamlessly integrate eSource and other 3rd party data sources to reduce Site Burden and improve data management.
Global security and compliance








Book a Demo →
Start and migrate your trials today – scale for tomorrows novel therapies



